11th March Antibody Engineering 2024
More than 100 monoclonal IgG antibodies have been approved for treatment of a range of diseases, including cancer, chronic inflammation, diabetes, migraine, and cardiovascular disorders.
This has spurred an extensive interest in engineering of antibody formats with improved efficacy for both therapeutic and prophylactic use
While most antibodies are directed against haematological and solid tumours, development of antibodies to prevent or treat infectious and neglected diseases is a growing field, as exemplified by the availability of licensed antibodies against SARS-CoV-2 to slow COVID-19 progression. Tailored antibodies may also become important treatment options against the growing threat from antimicrobial resistant (AMR) bacteria.
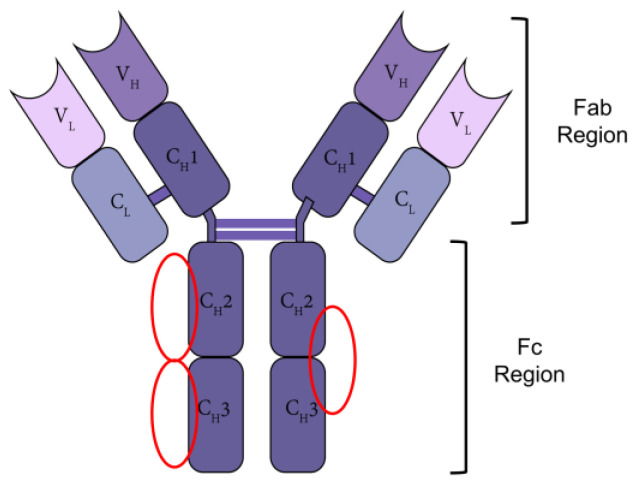
Most approved antibodies are built on a full length antibody protein, IgG1, the constant IgG1 Fc region is used as a fusion partner to extend tits present and kinetics. While natural IgG1 antibodies have a half-life of 3 weeks on average, individual monoclonal IgG1s have strikingly different half-lives, ranging from 6–32 days, while Fc-fusions mostly have shorter half-lives than IgG1.