Matters of the Heart and Immunology Systems
The body responds to various cardiac injuries via complex acute and chronic adaptive processes to maintain muscular pump function.
12th March 2024:
Central to this process is inflammation and immune cell signalling. For example, in the setting of acute ischemic injury, immune-mediated activation of cardiac fibrosis is necessary to avoid catastrophic myocardial rupture. Activation of resident interstitial fibroblasts and recruitment of those derived from the epicardium result in scar formation that maintains chamber integrity.
However, the initially beneficial fibroblast response can become maladaptive, resulting in excess ECM (extracellular matrix). This overaccumulation of ECM both stiffens the myocardium and negatively alters the cardiomyocyte niche resulting in progressive deterioration of cardiac function.
While fibroblast activation has been well studied, the multifaceted role of immune signalling and its effects on acute and chronic fibrosis remainsthe unknown with research ongoing.
The emerging understanding is poised to revolutionize the treatment of myocardial diseases through targeted immune modulators. Furthermore, advances in T-cell engineering offer exciting future directions for development of novel therapeutics.
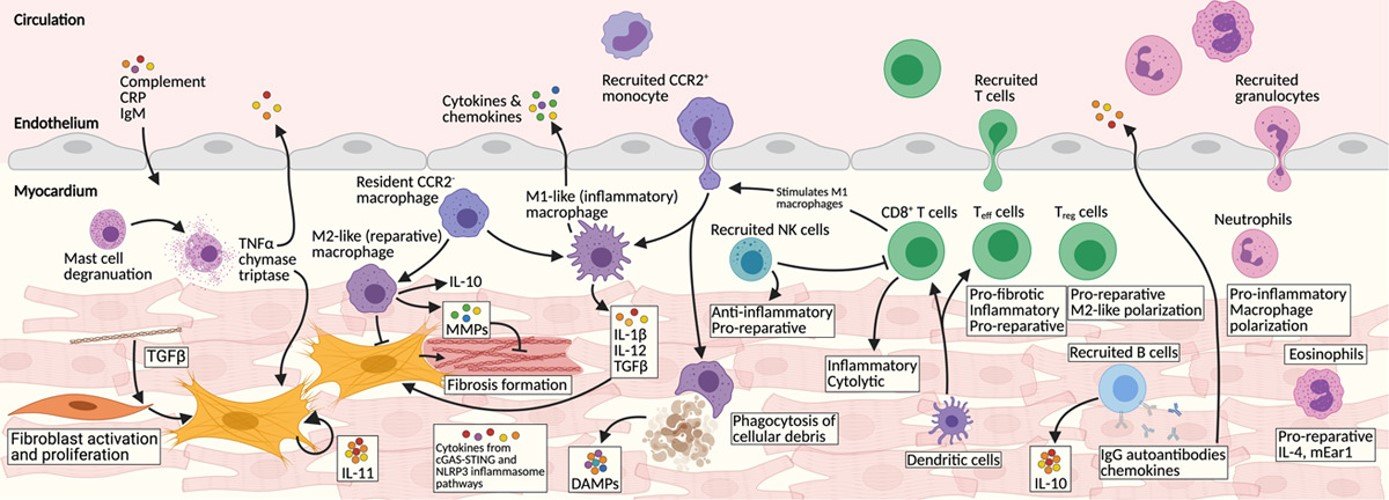
For further details see: Immune Cells and Immunotherapy for Cardiac Injury and Repair
The pathology of myocardial injury and repair is coordinated by a complex web of intersecting inflammatory pathways and immune cell types. Our understanding of the beneficial and harmful contributions of specific immune cells and cytokines is an exciting and emerging field of investigation. Targeted modulation of the immune system as a mechanism to boost myocardial recovery and repair is an important goal of cardio-immunology. M2-like macrophages, natural killer cells, and Tregs represent promising targets for therapeutic intervention. Furthermore, the prospect of engineering the immune system to reduce both fibrosis and senescence in aging and failing hearts offers potential future therapeutic avenues.
17th March 2024:
Ischemia/reperfusion injury–mediated (IRI-mediated) primary graft dysfunction (PGD) adversely affects both short- and long-term outcomes after lung transplantation, a procedure that remains the only treatment option for patients suffering from end-stage respiratory failure.
👉While B cells are known to regulate adaptive immune responses, their role in lung IRI is not well understood.
Here authors demonstrated by intravital imaging that B cells are rapidly recruited to injured lungs, where they extravasate into the parenchyma. Using hilar clamping and transplant models, lung-infiltrating B cells were observed to make a monocyte chemokine CCL7 in a TLR4-TRIF–dependent fashion, a critical step contributing to classical monocyte (CM) recruitment and subsequent neutrophil extravasation, resulting in worse lung function.
👉 They found that synergistic BCR-TLR4 activation on B cells is required for the recruitment of CMs to the injured lung.
👉 Finally, Farahnak et al corroborated findings in reperfused human lungs, observing a correlation between B cell infiltration and CM recruitment after transplantation.
This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Disclaimer. This post is written in a personal capacity only. A clinician should be consulted for further details.